Fluorination Portal
The efficient introduction of fluorine moieties into molecules has been at the forefront of research in the past few years. Extensive studies on the effect of fluorine atom on a molecule have shown enhanced biological activity, metabolic stability, binding interaction, and other favorable changes in physical properties of target compounds. However, the addition of fluorine to a molecule often requires harsh conditions and do not tolerate many functional groups. Working with world-class experts in fluorination chemistry, Aspira Scientific is pleased to offer single access to state-of-the art technologies, novel synthetic tools, and IP-enabling templates for drug discovery, crop protection, and material science. From gram-quantity catalog products to kilogram-and-beyond custom solutions, we bring the power of "fluorine" to your programs.
For custom solution inquiry, please contact collaborate@aspirasci.com.
F Addition
Fluolead™
Since 1978, Umemoto has been one of the pioneers in fluorine chemistry and contributed significantly to the expansion of the field, with the development of various popular fluorinating reagents that are used to this day. In 2010, Umemoto reported an air-stable alternative to common fluorinating agents such as DAST for the conversion of hydroxyl and carbonyl groups named Fluolead™.1 This reagent has proved to be very versatile reacting with a variety of hydroxyl and carbonyl groups yielding the corresponding fluorinated products in good to high yields. Fluolead™, a white to off-white crystalline material, also showed high thermal stability and high resistance to aqueous hydrolysis. Extensive studies have been conducted using DSC and ARC to evaluate the thermal stability of Fluolead™. These studies conclusively show the highest thermal stability of Fluolead™ compared to DAST and Deoxo-fluor™. In particular, it showed that Fluolead™ has an excellent thermal stability above 150 °C, which makes it the most stable deoxofluorination reagent to date. It gives Fluolead™ the ability to be used in reactions requiring temperatures in excess of 100 °C, commonly used for the conversion of carboxylic acids to trifluoromethyl groups. More importantly, these results illustrate the suitability of Fluolead™ for deoxofluorination reactions on commercial scales.
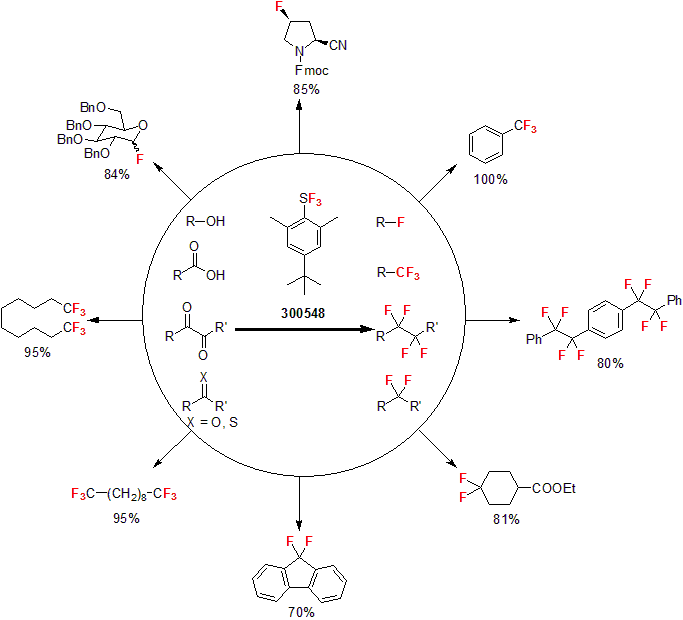
1 Umemoto, T. et al. J. Am. Chem. Soc. 2010, 132, 18199.
| Product # | Product Name | Structure |
|---|---|---|
| 300548 | Fluolead™ |  |
KF
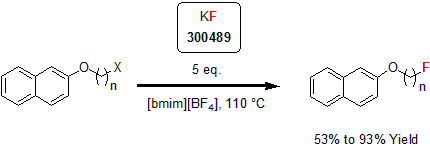
One of the first reagents that has been used for nucleophilic fluorination reaction is potassium fluoride. In the early reports in 1940s, the reaction conditions were very harsh with high temperatures and long reaction times.1 Coupled with its low solubility, its applications were very limited. Since these early reports, a wide variety of complementary reagents have been used to circumvent this issue, such as 18-crown-6, polymer supported fluoride or "spray-dried" KF.2 In 2002, Chi and co-workers reported a new method of fluorination in ionic liquids. In this report, the researchers highlighted the enhanced reactivity of potassium fluoride for the nucleophilic fluorination of halo- and mesyl-alkanes. Using ionic liquids allowed for lower reaction time and temperature.3
1 Forche, D. In Methoden der Organischen Chemie Houben-Weyl; Mueller, E., Ed.; George Thieme Verlag: Stuttgart, 1962; Vol. 5/3.
2 (a) Liotta, C. L.; Harris, H. P. J. Am. Chem. Soc. 1974, 96, 2250-2252. (b) Colonna, S. et al. J. Chem. Soc., Perkin Trans.1 1979, 2248-2252. (c) Ishikawa, N. et al. Chem. Lett. 1981, 761-764.
3 Kim, D. W. et al. J. Am. Chem. Soc. 2002, 124, 10278.
| Product # | Product Name | Structure |
|---|---|---|
| 300489 | Potassium fluoride, 99% |  |
SelectFluor®
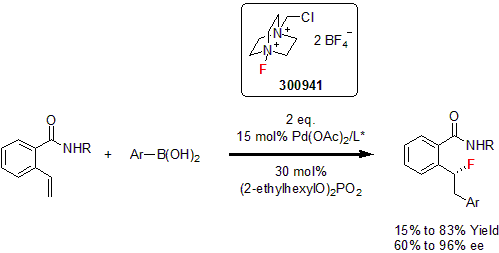
Electrophilic fluorination is one of the most effective methods to introduce the fluorine moiety into a molecule. However, a lot of the reagents available for this transformation, fluoroxy compounds or perchloryl fluoride can be very hazardous.1 In the early 1980's, a wide interest in N-F reagents was witnessed to palliate the lack of "user friendly" fluorination reagents.2 It is not until the early 1990's that Banks reported the synthesis and use of an air- and moisture-stable fluorination reagent based on triethyldiamine with a quarternary amine salt, named Selectfluor®.3 Selectfluor® is an effective fluorinating reagent for a wide variety of organic compounds such as hydrocarbons, aromatics, alkenes, carbohydrates, etc.4
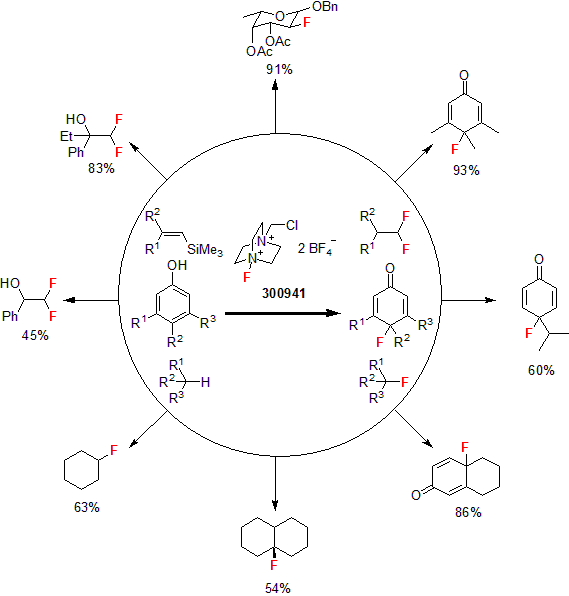
More recently, Toste and co-workers reported the asymmetric intermolecular fluorination of styrenes using Selectfluor® as the fluorinating agent. In this report, the researchers used a 3-component coupling of Selectfluor®, boronic acid, and styrene. A variety of enantio-enriched benzylic fluorides were synthesized in good yields and good enantiomeric excess.5
1 (a) Taylor, S. D. et al. Tetrahedron 1999, 55, 12431. (b) Bailey, W. H., III et al. Chem. Commun. 2002, 67, 1416. (c) Patrick, T. B. Electrophilic Fluorination of Carbon Hydrogen Bonds. In Chemistry of Organic Fluorine Compounds II: A Critical Review; Hudlicky, M., Pavlath, A. E., Eds.; ACS Monograph 187; American Chemical Society: Washington, DC, 1995; pp 133-171. (d) Rozen, S. et al. Chem. Rev. 1996, 96, 1717.
2 (a) Singh, S. et al. J. Am. Chem. Soc. 1987, 109, 7194. (b) Resnati, G. et al. J. Org. Chem. 1991, 56, 4925.
3 (a) Banks, R. E. J. Fluorine Chem. 1998, 87, 1. (b) Lal, G. S. et al. Chem. Rev. 1996, 96, 1737. (c) Furin, G. G. et al. Russ. Chem. Rev. 1999, 68, 653.
4 Shreeve, J. M. et al. Acc. Chem. Res. 2004, 37, 31.
5 Talbot, E. P. A. et al. J. Am. Chem. Soc. 2014, 136, 4104.
| Product # | Product Name | Structure |
|---|---|---|
| 300941 | 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate), 98% |  |
Fluoropyridinium Salts
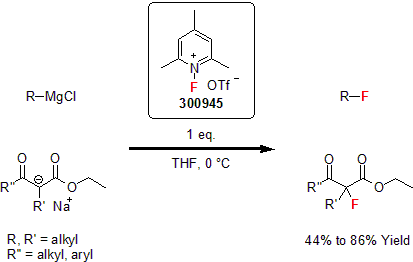
In 1986, Umemoto and co-workers reported the synthesis and application of N-fluoropyridinium salts. 1 These new salts were easy to handle with high reactivity. In their following work, they demonstrated the tunability of theses salts harnessing their reactivity and their selectivity. They showed that these reagents were efficient in the fluorination of various nucleophilic substrates such as vinyl esters, enol alkyl ethers, enol silyl ethers, enamines, and alkenes. Using mild reaction conditions, good to excellent yields were obtained. 2
More recently, Gouverneur and co-workers reported the asymmetric fluorocyclization of indenes using N-fluoro-2,6-dichloropyridinium triflate as their fluorination reagent. Various indenes were reacted to yield the corresponding fluorinated tetrahydro-5H-indeno[2,1-c]quinolones and hexahydrobenzo[k]phenanthridines in good to excellent yields as the syn diastereomers.3
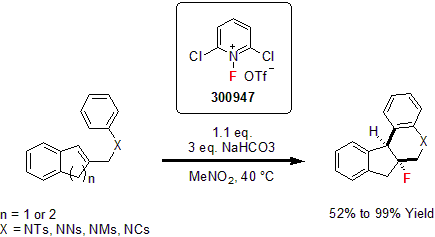
1 Umemoto, T. et al. Tetrahedron Lett. 1986, 27,4465. (b) Umemoto, T. et al. Bull. Chem. Soc. Jpn. 1986, 59, 3625.
2 Umemoto, T. et al. J. Am. Chem. Soc. 1990, 112, 8563.
3 Wolstenhulme, J. R. et al. Angew. Chem. Int. Ed. 2013, 52, 9796.
| Product # | Product Name | Structure |
|---|---|---|
| 300946 | 1-Fluoropyridinium triflate | 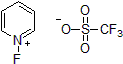 |
| 300947 | 2,6-Dichloro-1-fluoropyridinium triflate | 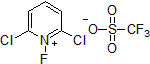 |
| 300945 | 1-Fluoro-2,4,6-trimethylpyridinium triflate | 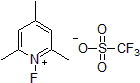 |
NFSI
In 1991, Differding and co-workers developed a new N-F sulfonyl based reagent, N-fluorobenzenesulfonimide (NFSI). They highlighted the reagent versatility in various fluorination transformations such as electrophilic fluorination of aromatics and of β-dicarbonyl and carbonyl compounds.1 More recently, Zhao and co-workers reported the ortho-selective C-H fluorination of oxalyl amide protected benzylamines. Using NFSI as the fluorinating reagent, a variety of oxalyl amide protected benzylamines were selectively mono- or di-fluorinated in good to excellent yields.2
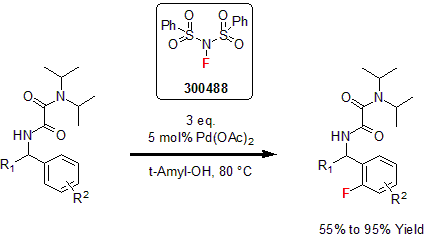
1 Differding, E. et al. Synlett 1991, 187.
2 Chen, C. et al. J. Org. Chem. 2014, 80, 942.
| Product # | Product Name | Structure |
|---|---|---|
| 300488 | N-Fluorobenzenesulfonimide, 97% | 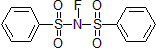 |
CF3 Addition
Trifluoromethylator®
Based on the cutting-edge technology developed by the John Hartwig group, one can simply add the CF3 moiety via a single reagent, Trifluoromethylator®;. This breakthrough reagent is very efficient not only with aryl iodide, but also with aryl bromide. The utility of the reagent was shown by reacting heteroaryl bromides and iodides with various functional groups, yielding the desired products in good to high yields.1 Finally, the versatility of Trifluoromethylator® was demonstrated by reacting a range of bromopyrazines, quinolones, quinoxolines, isoquinolines and aza-indoles affording the desired products in excellent yields.2
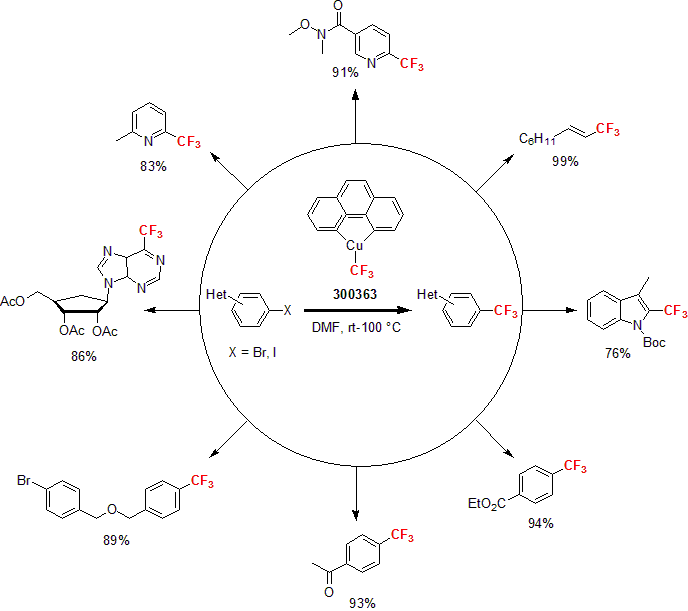
1 Morimoto, H. et al. Angew. Chem. Int. Ed. 2011, 50, 3793.
2 Mormino, M. G. et al. Org. Lett. 2014, 16, 1744.
Trifluoromethylator® Application Note
| Product # | Product Name | Structure |
|---|---|---|
| 300363 | Trifluoromethylator™ | 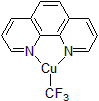 |
Pentafluoroethylator®
Following his report in 2011 of the Trifluoromethylator®, Hartwig and his group developed a new reagent for the addition of pentafluoroethyl group to various aryl bromides. Utilizing the Trifluoromethylator® framework, the Pentafluorethylator® is a higher order, copper-based complex delivering a pentafluoroethyl group to its targeted substrate. This breakthrough reagent allows scientists to generate various perfluoroethyl arenes and heteroarenes (Penta-F-Blocks™) from starting aryl and heteroaryl bromides; most of these compounds were not readily accessible by other chemistry previously. The typical reaction conditions for the Pentafluoroethylator® are similar to the Trifluomethylator®, using 1.2 equivalent of the reagent in DMF at 80 °C. Various aryl halides were fluorinated in good to excellent yields.1
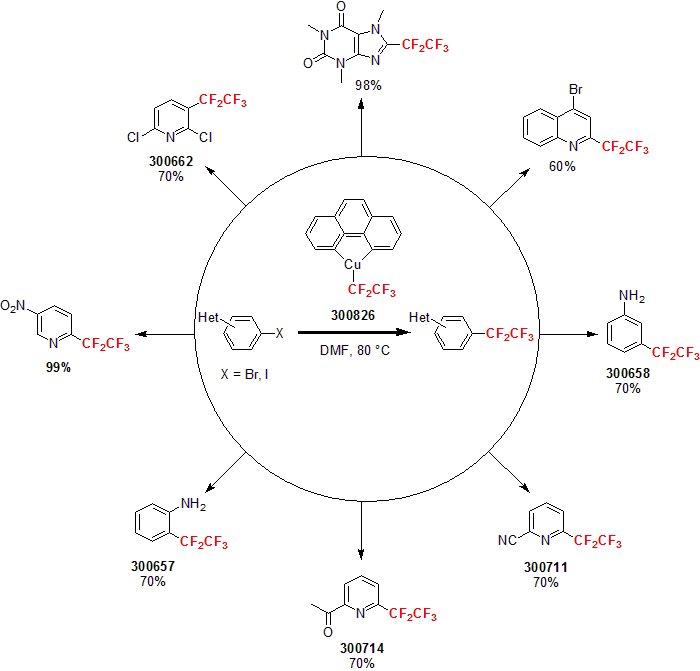
1 (a) Litvinas, N. et al. Angew. Chem. Int. Ed. 2012, 51, 536. (b) Mormino, M. G. et al. Org. Lett. 2014, 16, 1744.
Pentafluorethylator® Application Note
| Product # | Product Name | Structure |
|---|---|---|
| 300826 | (1,1,2,2,2-pentafluoroethyl)(1,10-phenanthroline-κN1,κN10)-copper, 95% | 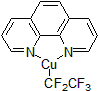 |
Ruppert-Prakash Type Reagents
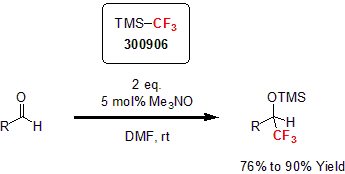
The pioneering work of Ruppert and Prakash which yielded the Ruppert-Prakash reagent (TMSCF3) was one of the first and most versatile ways to introduce a CF3 moiety by nucleophilic activation.1 In 2006, Prakash and his group reported the synthesis of TMS-protected trifluoromethylated alcohols using TMSCF3.2 Using trimethylamine N-oxide as the catalyst in tandem with TMSCF3 in DMF, the researchers highlighted the versatility of the synthetic method by reacting a variety of substituted aryl aldehydes and ketones. This method proved to be fast and insensitive to moisture for a variety of phosphates and carbonates yielding the corresponding TMS-protected alcohols in good to excellent yields.
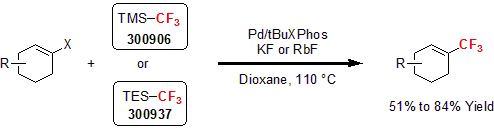
More recently, Buchwald and co-workers utilized several silicon-based CF3 reagents for the trifluoromethylation of various vinyl triflates and nonaflates.3 Using 8 to 12 mol% of palladium (II) complexes with tBuXPhos, 2 equivalents of TMSCF3 or TESCF3 with KF as an activator, a variety of vinyl triflates were trifluoromethylated in good to excellent yields.
1 (a) Ruppert, I. et al. Tetrahedron Lett. 1984, 24, 2195. (b) Prakash, G. K. S. et al. J. Am. Chem. Soc. 1989, 111, 393. (c) Prakash, G. K. S. et al. Chem. Rev. 1997, 97, 757.
2 Prakash, G. K. S. et al. J. Org. Chem. 2006, 71, 6806.
3 Cho, E. J. et al. Org. Lett. 2011, 13, 6552.
Pentafluorethylator® Application Note
| Product # | Product Name | Structure |
|---|---|---|
| 300815 | Trimethyl(trifluoromethyl)silane, 98% | 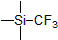 |
| 300906 | Trimethyl(trifluoromethyl)silane, 99% | 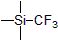 |
| 300937 | Trifluoromethyl triethylsilane, 98% | 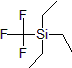 |
| 300814 | (Pentafluoroethyl)trimethylsilane, 97% | 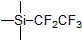 |
Togni Reagents
In 2006, Togni and his group reported the first electrophilic trifluoromethylation via a hypervalent iodine reagent.1 This new reagent based on a λ3-iodane core is capable of transferring an electrophilic CF3 unit. This air-stable, crystalline solid proved to be a very versatile reagent for the trifluoromethylation of a variety of alcohols, thiols, phosphines, and secondary amines.2
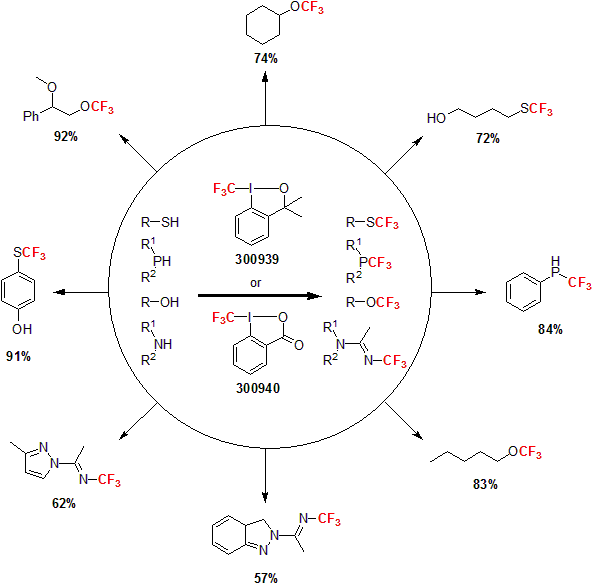
1 Eisenberg, P. et al. Chem. Eur. J. 2006, 12, 2579.
2 Charpentier, J. et al. Chem. Rev. 2015, 115, 650.
| Product # | Product Name | Structure |
|---|---|---|
| 300939 | Trimethyl(trifluoromethyl)silane, 98% | 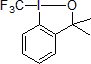 |
| 300940 | Trimethyl(trifluoromethyl)silane, 99% | 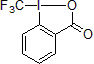 |
Sodium Sulfinates
In 1991, Langlois and co-workers were able to generate a trifluoromethane radical under oxidative conditions using sodium trifluoromethanesulfinate as a precursor.1 For the next 20 years, this reagent was infrequently used with mixed successes. It was not until 2011, when Phil Baran and his group successfully used the Langlois reagent in the C-H trifluoromethylation of N-heterocyclic compounds that broadened its applicability and increased its usage in literature.2 This metal-free reaction can be applied to unprotected molecules with limited side reactions.
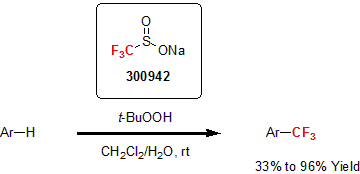
Since this report, publications have flourished demonstrating the versatility of the Langlois reagent for the trifluoromethylation of aromatics, alkenes, and alkynes.3 In particular, Hu and his group recently demonstrated the utility of these reagents as efficient radical fluoroalkylating reagents able to react with conjugated N-arylsulfonated amides to yield the desired fluoroalkylamides.4
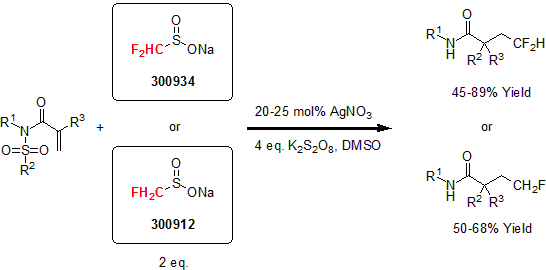
1 Clavel, J.-L. et al. Phosphorus, Sulfur, Silicon Relat. Elem. 1991, 59, 169.
2 (a) Ji, Y. et al. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 14411. (b) Musumeci, D. et al. Chem. Commun. 2013, 4, 1405.
3 (a) Cao, X.-H. et al. Chem. Commun. 2014, 50, 3359. (b) Yang, Y.-D. et al. Chem. Commun. 2013, 49, 5510. (c) Li, Y. et al. Chem. Commun. 2013, 49, 2628. (d) Jiang, X.-Y. et al. Angew. Chem., Int. Ed. 2013, 52, 14177. (e) Deb, A. et al. Angew. Chem., Int. Ed. 2013, 52, 9747. (f) Cui, L. et al. Adv. Synth. Catal. 2013, 355, 2203.
4 He, Z. et al. Org. Lett. 2015, ASAP.
| Product # | Product Name | Structure |
|---|---|---|
| 300942 | Sodium trifluoromethanesulfinate, 97% | 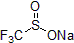 |
| 300934 | Sodium difluoromethanesulfinate, 97% | 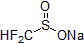 |
| 300912 | Sodium fluoromethanesulfinate, 97% | 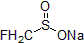 |
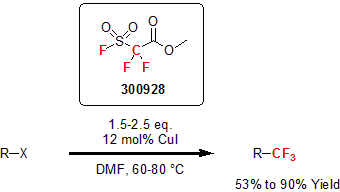
Fluorosulfonyl Reagents
In 1989, Chen and Wu reported the use of fluorosulfonyldifluoroacetate (also known as the Chen reagent or MFDA) as a reagent for the trifluoromethylation of a wide range of organohalides. Using CuI in a catalytic amount in DMF, a variety of alkenes, aromatics, and amides were functionalized with trifluoromethane in good to excellent yields. This reagent proved to be low cost, very stable, and convenient to use.1
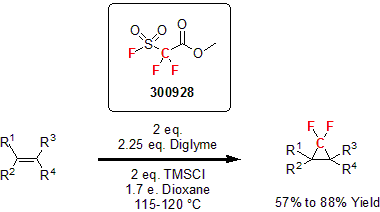
More recently, Dolbier and his group published the use of MFDA in the synthesis of various difluorocyclopropane molecules. In this study, the researchers shows that under certain conditions, MDFA acts as an efficient source of difluorocarbene. A variety of substituted alkenes were reacted yielding the desired gem-difluorocyclopropanes in good to excellent yields.2
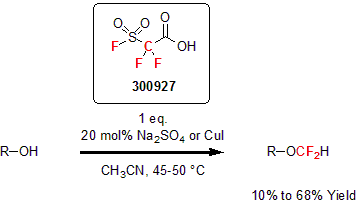
Also developed by Chen and his group, the β-sultone derivative fluorosulfonyldifluoroacetic acid proved to be an effective difluorocarbene source in acids. This reagent can difluoromethylate alcohols and phenols. In a typical reaction, a catalytic amount of sodium sulfate or copper iodide is used in acetonitrile to yield the corresponding ethers in good yields.3
1 Chen, Q.-Y. et al. Chem. Commun. 1989, 705.
2 Eusterwiemann, S. et al. J. Org. Chem. 2012, 77, 5461.
3 Chen, Q.-Y. et al. J. Fluorine Chem. 1989, 44, 433.
| Product # | Product Name | Structure |
|---|---|---|
| 300928 | Methyl 2,2-difluoro-2-(fluorosulfonyl)acetate, 97% | 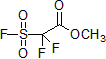 |
| 300927 | 2,2-Difluoro-2-(fluorosulfonyl)acetic acid, 97% | 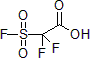 |
CH2CF3 Formation
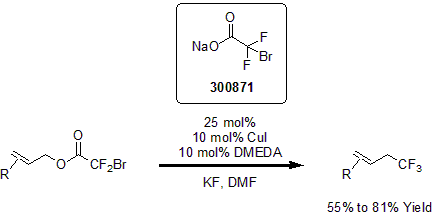
In 2013, Altman and his group reported the copper-catalyzed decarboxylative trifluoromethylation of various allylic alcohols. Using various allyl alcohols as precursors, the researchers were able to convert them to trifluoromethane in 2 steps, when previous methods required at least 4 steps. In this report, using copper iodide as a catalyst with DMEDA and sodium bromodifluoroacetate, various allyl alcohols were converted to the corresponding trifluoromethanes via bromo difluoroacetic ester intermediates, in good yields.1
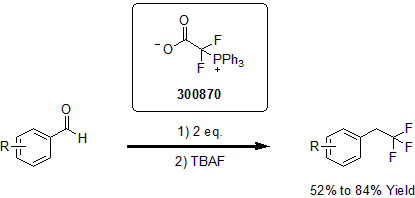
In a following report in 2014, Altman described the metal-free trifluoromethylation of aromatic and heteroaromatic aldehydes and ketones. In this one pot procedure, (triphenylphosphonio)difluoroacetate and TBAF are used as reagents for the rapid and efficient conversion of various aryl and heteroaryl aldehydes and ketones into the corresponding trifluoroarenes in good yields.2
1 Ambler, B. R. et al. Org. Lett. 2013, 15, 5578.
2 Qiao, Y. et al. J. Org. Chem. 2014, 79, 7122.
| Product # | Product Name | Structure |
|---|---|---|
| 300871 | Sodium bromodifluoroacetate | 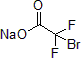 |
| 300870 | (Triphenylphosphonio)difluoroacetate | 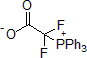 |
CF2 Addition
TMSCF2H Type Reagents
In 2011, Hu and his group developed an efficient difluoromethylation of carbonyl compounds and imines.1 In this report, they used TMSCF2H as a nucleophile activated by a Lewis base at room temperature. This procedure proved to be successful with a variety of ketones, aldehydes, and imines with high yields of the corresponding fluorinated product.
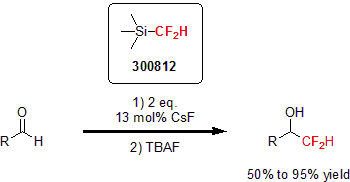
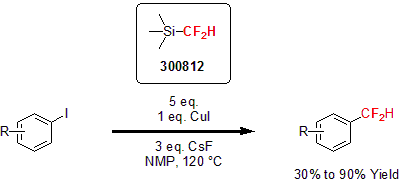
Using TMSCF2H in 2012, Hartwig and his group developed a copper-mediated difluoromethylation of aryl and vinyl iodides.2 They showed the versatility of this reaction by reacting electron-neutral, electron-rich, and sterically hindered aryl and vinyl iodides with CuI, CsF, and TMSCF2H to yield the difluoronated product in high yields. In addition, the reaction tolerates a wide variety of functionalities such as amines, ethers, amides, and esters. In a typical reaction, 1 equivalent of CuI, 3 equivalents of CsF and 5 equivalents of TMSCF2H are combined with the substrate in NMP at 120 °C for 24 hours.
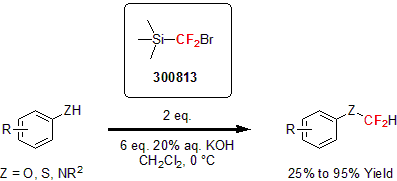
Hu and his group developed another fluorination reagent for the difluoromethlyation of heteroatom nucleophiles and the difluoromethylenation of alkenes and alkynes.3 This new reagent proved to be a safer alternative to TMSCF2H since no gaseous by-product is generated. The reagent underwent addition with various alkynes and alkenes, affording difluoromethyl-containing cyclopropenes and cyclopropanes, respectively.
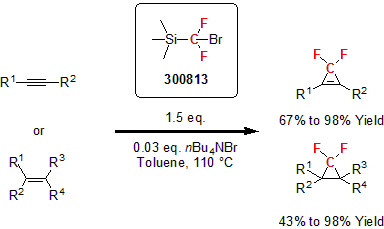
In addition, the reagent reacted with O, S, and N nucleophiles exhibiting good tolerance of different functionalities.
1 Zhao, Y. et al. Org. Lett. 2011, 13, 5342.
2 Fier, P. S. et al. J. Am. Chem. Soc. 2012, 134, 5524.
3 Li, L. et al. Angew. Chem. Int. Ed. 2013, 52, 12390.
| Product # | Product Name | Structure |
|---|---|---|
| 300812 | Difluoromethyltrimethylsilane, 97% | 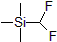 |
| 300813 | (Bromodifluoromethyl)trimethylsilane, 98% | 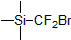 |
| 300936 | (Chlorodifluoromethyl)trimethylsilane, 97% | 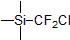 |
Bromodifluoromethylcarboxylic Acid Derivatives
The Xingang Zhang group recently developed an efficient method for difluoroalkylation via palladium catalysis.1 This method features several advantages including broad substrate scope, excellent functional group compatibility, and synthetic simplicity. This process provides access to novel difluoromethylated carboxylic acid derivatives and phosphonates in good yields.
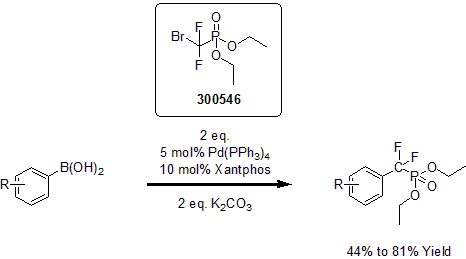
1 Zhang, X. et al. Angew. Chem. Int. Ed. 2014, 53, 1669.
| Product # | Product Name | Structure |
|---|---|---|
| 300545 | Ethyl bromodifluoroacetate, 97% | 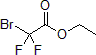 |
| 300547 | 2-Bromo-2,2-difluoro-1-morpholinoethanone, 97% | 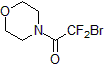 |
| 300546 | Diethyl (bromodifluoromethyl)phosphonate, 97% | 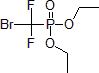 |
| 300812 | Difluoromethyltrimethylsilane, 97% |  |
Aryl and Heteroarylsulfonyl Reagents
First reported by Hine and Porter in 1960, difluoromethyl phenyl sulfone became one of the most popular reagent used for nucleophilic difluoro(phenylsulfonyl)methylation.1 This reagent allows the introduction of the CF2H moiety via the reduction of the sulfone. Furthermore, the phenylsulfonyl group is a more effective activation group to introduce the difluoromethyl group, making this reagent very effective. Over the years, several reports highlighted the versatility of this reagent with various ketones and aldehydes.2 In a typical reaction, a strong base is used such as LiHMDS; the combination of N(TMS)3 and Me4NF or KOH is used to activate the sulfone moiety for the nucleophilic addition to the carbonyl group. The sulfone is then reduced by a metal reductant to yield the desired difluoromethyl moiety.3
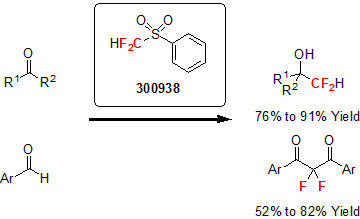
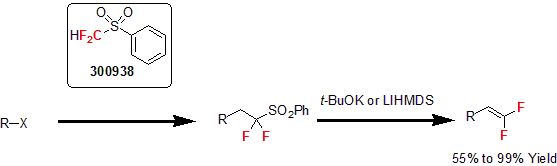
More recently, the Prakash group reported the synthesis of gem-difluoroalkenes using difluoromethyl phenyl sulfone for the difluoro(phenylsulfonyl)methylation of primary alkyl iodides or bromides followed by their dehydrosulfination.4 Following this report, several researchers used a similar method for the synthesis of various gem-difluorinated allyl alcohols, amines, 1,3-dienes, and enol esters.5
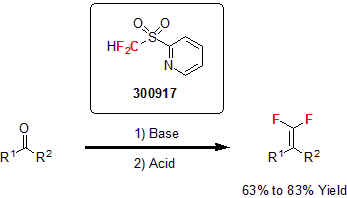
In 2010, Hu and co-workers reported the synthesis of 1,1-difluoroalkenes from carbonyl compounds using the newly developed 2-pyridyl sulfone, a bench-stable, crystalline solid. Using a similar procedure to its parent reagent, difluoromethyl phenyl sulfone, various ketones and aldehydes were difluoro-olefinated using a base, followed by the treatment with an acid to yield the corresponding 1,1-difluoroalkenes in good yields.6
1 Hine, J.; Porter, J. J. J. Am. Chem. Soc. 1960, 82, 6178.
2 (a) Stahly, G. P. J. Fluorine Chem. 1989, 43, 53. (b) Prakash, G. K. S. et al. Angew. Chem., Int. Ed. 2003, 42, 5216. (c) Zhu, L. et al. J. Fluorine Chem. 2007, 128, 1098.
3 (a) Sabol, J. S. et al. Tetrahedron Lett. 1992, 33, 3101. (b) Prakash, G. K. S. et al. Eur. J. Org. Chem. 2005, 2218.
4 Prakash, G. K. S. et al. Angew. Chem., Int. Ed. 2004, 43, 5203.
5 (a) Ni, C. et al. Angew. Chem., Int. Ed. 2006, 46, 786. (b) He, Z. et al. Angew. Chem., Int. Ed. 2012, 51, 11545.
6 Zhao, Y. et al. J. Org. Lett. 2010, 12, 1444.
| Product # | Product Name | Structure |
|---|---|---|
| 300938 | Phenyl difluoromethylsulfone, 98% | 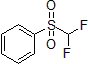 |
| 300917 | 2-Pyridyl difluoromethanesulfone, 97% | 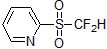 |
(SIPr)AgCF2H
More recently, Shen and co-workers reported the cooperative dual palladium/silver catalyst for the difluoromethylation of aryl bromides and iodides. This transformation, usually done under harsh conditions and with a stoichiometric amount of catalysts, is accomplished using a catalytic amount of a palladium/silver catalyst system. The researchers demonstrated the versatility of this method with the difluoromethylation of various aryl bromides and iodides using 5-7 mol% of the palladium precursor, with 10-14 mol% of the dppf ligand and 20 mol% of the silver complex, with 2 equivalents of TMSCF2H and NaOtBu. This method proved to be compatible with various functional groups such as esters, amides, protected phenoxides, protected ketones, pyridines or cabazoles affording the desired fluorinated aryl product in moderate to excellent yields.1
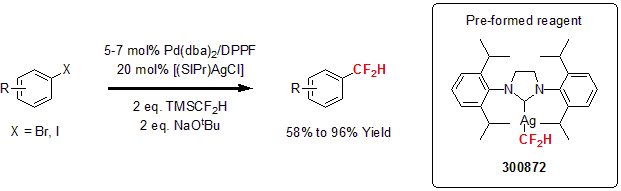
1 Gu, Y. et al. Nature Comm. 2014, 5, 5405.
| Product # | Product Name | Structure |
|---|---|---|
| 300872 | [1,3-Bis[2,6-bis(1-methylethyl)phenyl]-2-imidazolidinylidene]difluoromethyl silver | 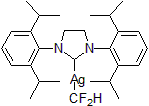 |
SCF3 Addition
(bpy)CuSCF3
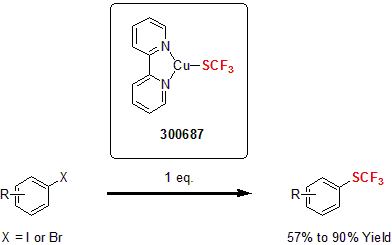
Compounds containing trifluoromethylthio groups have attracted increasing attention in the past few years due to their high lipophilicity and hydrophobicity. However, the synthetic tools to access this moiety are fairly limited and often requires high reaction temperatures and the use of expensive and/or toxic reagents. Recently, Weng and co-workers addressed these drawbacks by developing an air-stable copper reagent for the nucleophilic trifluoromethylthiolation of various aryl halides. The bipyridine copper complex proved to be the most efficient in this transformation for various aryl halides into the corresponding trifluoromethylthiolated product in modest to good yields.1
Thiosaccharin SCF3 Reagent
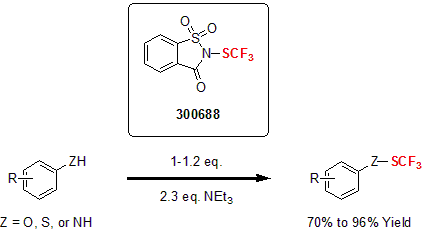
More recently, Shen and his group developed a N-trifluoromethylthiosaccharin reagent for the electrophilic trifluoromethylthiolation of a variety of nucleophiles. This reagent proved to be very versatile converting a variety of substrates such as alcohols, aldehydes, ketones, electron-rich arenes and alkynes into the corresponding trifluoromethylthiolated product in good yields under mild conditions.2
1 Weng, Z. et al. Angew. Chem. Int. Ed. 2013, 52, 1548.
2 Xu, C. et al. Angew. Chem. Int. Ed. 2014, 53, 9316.
| Product # | Product Name | Structure |
|---|---|---|
| 300687 | (2,2'-bipyridine-κN1,κN1')(1,1,1-trifluoromethanethiolato-κS), 97% | 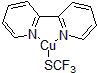 |
| 300688 | 1,1-Dioxo-2-trifluoromethylsulfanyl-1,2-dihydro-1l6-benzo[d]isothiazol-3-one, 97% | 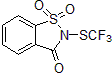 |
FAYE Blocks
Inspired by the antidiabetic drug, Januvia®, and the needed access for polyfluoroaromatics, Weaver and his group reported the synthesis of various perfluoro and polyfluoroaryl Meldrum acids. These innovative building blocks, FAYE Blocks (Fluoroaryl AcYl Equivalents), give access to a variety of air-stable fluorinated aromatics that can be readily derivatized through the Meldrum’s acid handle.1 Further downstream manipulations such as hydrodefluorination lead to polyfluoroaromatics and heteroaromatics in rapid order.2
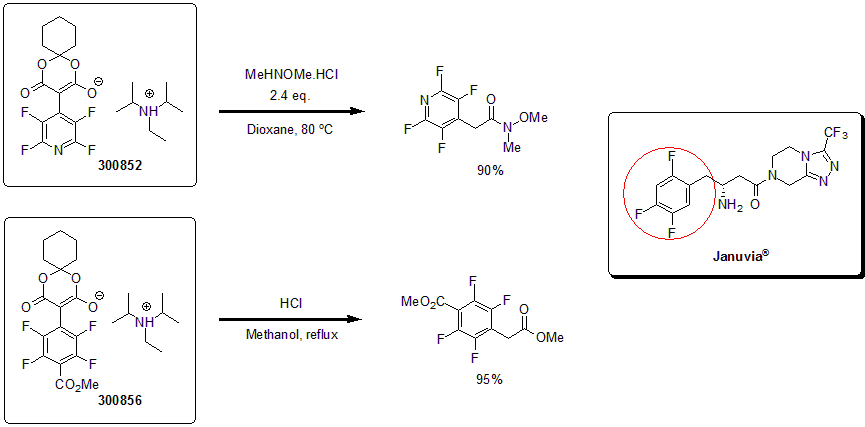
1 Senaweera, S. M.; Weaver, J. D. J. Org. Chem. 2014, 79, 10466.
2 Senaweera, S. M. et al. J. Am. Chem. Soc. 2014, 136, 3002.
Click here to see a list of FAYE Blocks.
SF5 Compounds
The SF5 moiety has been called the Substituent of the Future because of its enhanced lipophilicity, electronegativity, and metabolic stability. It has been used in several drugs and crop protective agents and showed improved efficacy in a number of cases. Aspira Scientific is pleased to offer a series of SF5 compounds to accelerate your drug and material development programs.
Click here to see a list of SF5 Compounds.
For custom solution inquiry, please contact collaborate@aspirasci.com.

